A Novel 7-O-glucosyltransferase Isolated from P. lobata
2014-04-15
Glycosides are natural perssades
Pueraria lobata roots have been ascribed a number of pharmacological properties, including hypolipidemic and hypertension effects, anti-tumor activities, preventing osteoporosis and other medicinal effects. Most pharmacological activities of P. lobata extracts are due to the presence of isoflavone O-glycosides, such as daidzin, genistin and ononin. The glucosyltransferase gene isolated from P. lobata holds a potential in the process of either synthesizing novel drugs or glycosylating the existing medicinal compounds.
To investigate the isoflavone metabolism in P. lobata, a research team led by Prof. ZHANG Yansheng at Wuhan Botanical Garden isolated seven full-length UGT candidates with preferential expression in roots. Functional assays in yeast revealed that PlUGT1 (official UGT designation UGT88E12), efficiently glycosylated isoflavone aglycones at the 7-hydroxy group. Recombinant PlUGT1 purified from Escherichia coli cells was shown to be relatively specific for isoflavone aglycones, while flavonoid substrates were poorly accepted. The biochemical results suggested that PlUGT1 was an isoflavone 7-O-glucosyltransferase. The transcript abundance of PlUGT1 was correlated with the accumulation pattern of isoflavone glycosides such as daidzin in P. lobata plants or in cell suspension culture. The biochemical properties and gene expression profile supported the idea that PlUGT1 could play a role in isoflavone glycosylation in P. lobata.
Relevant results were published in Plant Cell Reports online entitled “Molecular cloning and characterization of an isoflavone 7-O-glucosyltransferase fromPueraria lobata”.
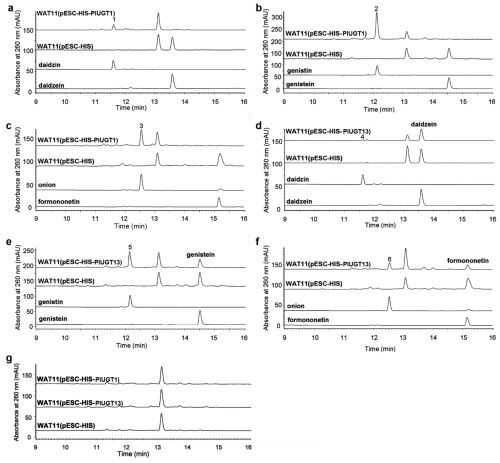
Functional characterization of glucosyltransferases in yeast (Image by WBG)