Name:ZHANG Yansheng
Tell:
Email:zhangys@wbgcas.cn
Organization:Wuhan Botanical Garden
Researchers Discover the Mechanism underlying the C12,8-lactone Moiety Formation
2017-12-07
Sesquiterpene lactones (STLs) are a large class of secondary metabolites from plants with various pharmacological activities. A significant example is the sesquiterpene lactone thapsigargin, which is produced by Thapsia garganica L. A thapsigargin, derived-drug towards prostate cancer tumors is currently in clinical trials.
The major activities of STLs are due to the presence of sesquiterpene lactone ring moieties in their molecules. Based on the regio-configurations of the lactone ring groups, these STLs can be classified into two types of C12,6 and C12,8. The knowledge regarding the biosynthesis of the C12,6-lactone ring has been established so far, while how the C12,8-lactone moiety is formed is not clear.
A project aiming to understand the mechanism underlying the formation of the C12,8-lactone moiety was launched by the Natural Product Biosynthesis Laboratory of the Wuhan Botanical Garden. Under the supervision of Prof. ZHANG Yansheng , the Ph.D student GOU Junbo has successfully isolated a cytochrome P450 gene CYP71BL6 from Inula Hupehensis plant. TheCYP71BL6 introduced a hydroxyl group to the C-8 position of germacrene A acid, and the resulting 8α-hydroxyl germacrene A acid spontaneously lactonized to a C12,8α-lactone.
The identification of CYP71BL6 provides a potential to synthesize the C12,8-STLs in microbial hosts by synthetic biology. To implement this possibility, GOU Junbo has constructed the whole germacrene A-type pathway in yeast cells through germacrene A acid followed by the oxidative reaction by CYP71BL6. This engineered yeast strain was able to produce the C12,8-STL inunolide when fed with a normal yeast medium.
This finding has been published in Plant Journal entitled “Discovery of a non-stereoselective cytochrome P450 catalyzing either 8α- or 8β-hydroxylation of germacrene A acid from the Chinese medicinal plant,Inula hupehensis”. It was supported by the National Natural Science Foundation of China.
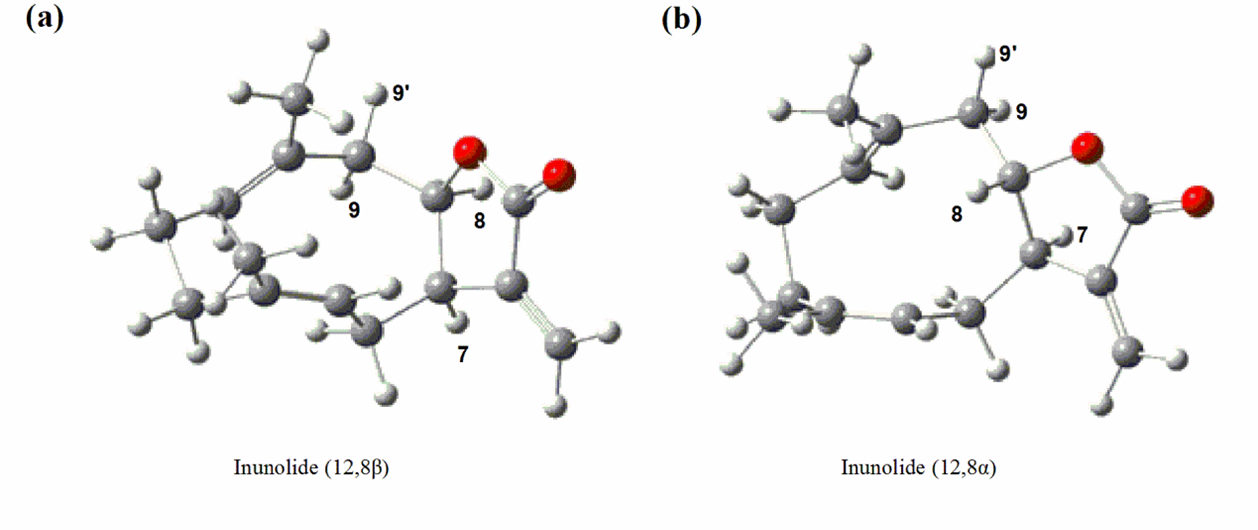
The stereo-selective formation of the C12,8-lactone ring by CYP71BL6 (Image by GOU Junbo)
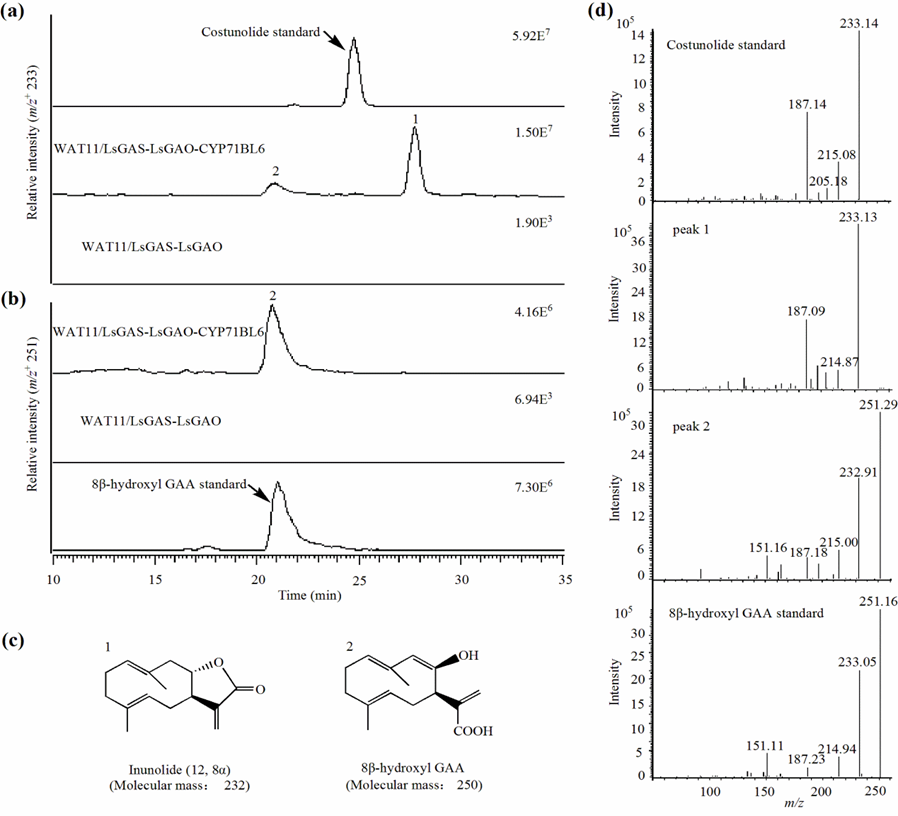
The synthesis of the C12,8-STL inunolide in an engineered yeast strain using CYP71BL6 (Image by GOU Junbo)